Soap is a part of our daily life, it has different shapes and perfumes, it can be solid or liquid... But have you ever thought about how this common substance works? Here is a little explanation. I've used a minimum number of scientific terms, so I hope that everybody can understand it.
There are substances which can be dissolved in water (salt for example), and others that can't (for example oil). Water and oil don't mix together, so if we try to clean an oily stain from a cloth or from the skin, water is not enough. We needsoap.
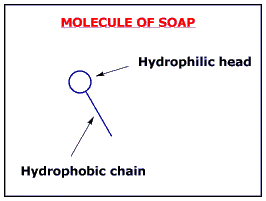
Soap is formed by molecules with a "head" which likes water (hydrophilic) and a long chain which hates it(hydrophobic).
Because of that dualism, soap molecules act like a diplomat, improving the relationship between water and oil. How? When soap is added to the water, the hydrophilic heads of its molecules stay into the water (they like it!), while the long hydrophobic chains join the oil particles and remain inwards (escaping from the water). In that way, they form circular groups named micellas, with the oily material absorbed inside and trapped.
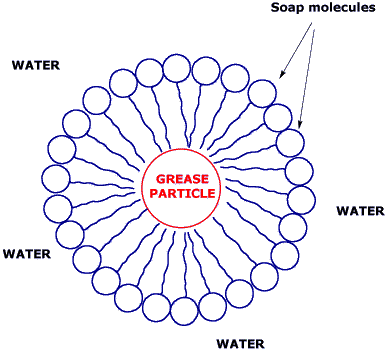
An emulsion of oil in water is then formed, this means that the oil particles become suspended and dispersed into the water. Thus, those oil particles are liberated from the cloth or the skin, and the emulsion is taken away with the rinsing.
In summary, soap cleans by acting as an emulsifier. It allows oil and water to mix so that oily grime can be removed during rinsing. There are more things involved in this process, but this is the general idea.
.
 Kuala Lumpur Time
Kuala Lumpur Time
0 comments:
Post a Comment