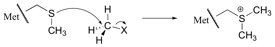Introduction:
Substitution, addition, and elimination reactions are of great importance in a major branch of chemistry known as Organic Chemistry, which covers the chemistry of compounds of carbon. These reactions, which generally involve covalently bonded molecules, are also found, to a much more limited extent with other compounds.
Substitution reactions:
A substitution reaction is a reaction in which an atom (or group of atoms) in a molecule is replaced by another atom or group of atoms:
Example 1:
The gas ethane, CH3CH3 reacts with bromine vapour in the presence of light to form bromoethane, CH3CH2Br and hydrogen bromide, HBr. In the process, a hydrogen atom in ethane has been substituted for a bromine atom:
Example 2:
Ethanol, CH3CH2OH, reacts with hydrogen iodide, HI, to form iodoethane and water. Here, a group of atoms, OH, has been replaced by an iodine atom:
Example 3:
Benzene, C6H6, reacts with bromine in the (presence of iron bromide as catalyst) to form bromobenzene, C6H5Br. This results in a hydrogen atom being replaced by a bromine atom:
Addition reactions:
An addition reaction is a reaction whereby a molecule reacts with another molecule having one or more multiple covalent bonds so as to form a molecule whose molecular mass is the sum of the molecular masses of the reacting molecules:
Example 3:
Ethene, CH2=CH2 has a double bond joining the two carbon atoms. This substance can add a hydrogen molecule (in the presence of platinum as catalyst) to form ethane, CH3CH3:
Example 5:
Ethyne, C2H2 has a triple bond joining the two carbon atoms. Hydrogen bromide adds onto this triple bond to form 1,1-dibromoethane, CH3CHBr2:
Elimination reactions:
An elimination reaction is a reaction whereby a multiple covalent bond is formed in a molecule by the removal of another, usually smaller molecule:
Example 6:
Ethanol, CH3CH2OH, when treated with concentrated sulphuric acid, H2SO4, loses 2 hydrogen atoms and one oxygen atom, forming ethene, CH2=CH2 and water (the atoms that have been eliminated are shown in red):
When an elimination reaction removes the elements of water from a compound, as in the reaction above, the reaction is called a DEHYDRATION REACTION.
Example 7:
Bromoethene, CH2=CHBr, when treated with potassium hydroxide dissolved in ethanol, loses one hydrogen atom and one bromine atom, forming ethyne, CH≡CH (the atoms that have been eliminated are shown in red):
When an elimination reaction removes the elements of a halogen acid (HCl, HBr, HI) from a compound, as in the reaction above, the reaction is called a DEHYDROHALOGENATION REACTION.

 Kuala Lumpur Time
Kuala Lumpur Time